Abstract
Background: Prior studies have suggested that achieving CR is critical to outcome in frontline therapy of PTCL. In clinical practice, the decision of whether to recommend HSCT for PTCL patients in first CR is one that is routinely faced. However, data to guide the decision are limited, and clinical practice is therefore predicated on lower level evidence. Phase 2 studies have reported encouraging outcomes associated with HSCT in the frontline setting; however, comparing results of these prospective studies to historic controls is hindered by diverse eligibility criteria, possible selection bias, and differing rates of CR prior to HSCT. Furthermore, the survival data from most phase 2 studies do not include patients with chemoresistant disease (i.e. less than partial or complete response after induction chemotherapy), thereby inherently biased towards a selected group of patients with more favorable prognosis. In the present study, we seek to understand whether HSCT confers better survival outcomes in PTCL patients in first CR. Our analyses were performed on patients enrolled in COMPLETE (Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment), a large prospective multicenter cohort study of newly diagnosed PTCL patients in the United States.
Methods: Patients were eligible for enrollment in COMPLETE if they had a new diagnosis of histologically confirmed aggressive subtypes of PTCL. Patients were included in this analysis if their best response to induction therapy was a CR. Survival-based analyses were performed using Kaplan-Meier methodology. Multivariable Cox regression was used to assess the relationship between pre-specified variables and survival.
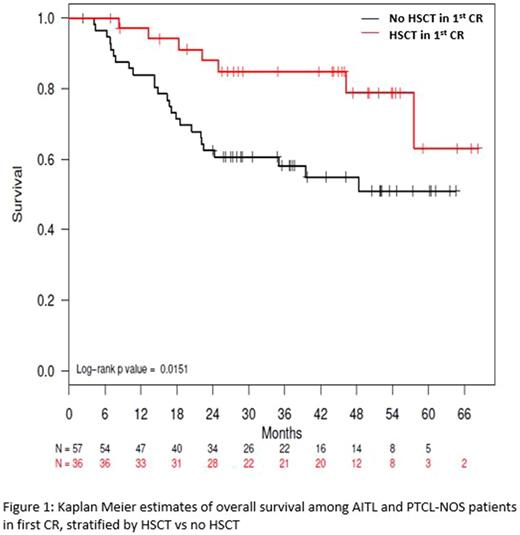
Results: A total of 499 PTCL patients were enrolled in COMPLETE over 4 years. Two hundred thirteen patients achieved a CR following frontline therapy and had the required locked records for the analysis with a median follow-up of 2.9 years (Range 1.9-4.0). One hundred forty nine (70%) of these received induction therapy without HSCT consolidation and 64 (30%) underwent either autologous (n = 49) or allogeneic (n = 15) HSCT. Response was determined by PET/CT scans in 125 (59%) patients, and the rest (41%) were done by CT, MRI, and/or PET alone or in combination. Although there were no significant differences in demographics (age, gender, and performance status) between the HSCT vs. no-HSCT groups, there were significantly more patients with advanced stage (III/IV) disease and IPI ≥ 2 in the HSCT group. Among patients with angioimmunoblastic T-cell lymphoma (AITL, n = 35) and PTCL-Not-Otherwise-Specified (PTCL-NOS, n = 58), there was a significant difference in Progression Free Survival (PFS) in favor of HSCT with median PFS of 57.6 months compared to 25.5 months in the non-HSCT group (P = 0.05). Overall survival (OS) also favored the HSCT with median survival not reached in either group in patients with AITL and PTCL-NOS (Figure 1, P = 0.02). The survival advantage of HSCT became less evident when anaplastic large cell lymphoma (ALCL, n = 51) was added to the analysis with median OS and PFS rates not reached in either group (P= 0.06 and 0.17, respectively). Of note, both ALK-positive (n = 21) and negative (n = 30) ALCL were included in the analysis. In an exploratory multivariate analysis of patients with the diagnosis of nodal PTCL, including AITL, ALCL, and PTCL-NOS, HSCT was associated with superior overall survival with a hazard ratio of 0.26 (0.10 - 0.68; P < 0.01) when controlled for the staging and IPI scores.
Conclusions: This is the first report from the largest prospective PTCL database in the U.S. to date to examine the role of HSCT in PTCL patients who are in first CR. Our data demonstrate potential survival advantages of upfront HSCT in patients with nodal PTCL who achieve CR. However, the results should be interpreted with caution given a relatively short median follow up as well as the non-randomized study design. The role of HSCT among various groups of PTCL patients in first CR should further be evaluated in randomized controlled trials.
Park: Takeda: Research Funding; Seattle Genetics: Research Funding; Gilead: Speakers Bureau; Teva: Consultancy, Research Funding; Cornerstone Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Horwitz: Celgene: Consultancy, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Mundipharma: Consultancy; ADCT Therapeutics: Research Funding; Infinity/Verastem: Consultancy, Research Funding; Aileron Therapeutics: Research Funding; BMS: Consultancy; HUYA: Consultancy; Forty-Seven: Consultancy, Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding. Foss: celgene: Honoraria; immune design: Research Funding; spectrum: Honoraria, Speakers Bureau; Eisai: Honoraria; seattle genetics: Speakers Bureau. Carson: Seattle Genetics: Honoraria. Pro: Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy. Hsi: Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Abbvie: Research Funding; Eli Lilly and Co.: Research Funding; Cellerant Therapeutics: Research Funding. Federico: takeda: Honoraria, Research Funding. Schwartz: MedNet Solutions: Employment. Bellm: MedNet Solutions: Employment. Advani: Seattle Genetics: Research Funding; Kura: Research Funding; Millennium: Research Funding; Merck: Research Funding; Pharmacyclics: Consultancy; Pharmacyclics: Research Funding; Sutro: Consultancy; Infinity: Research Funding; Celgene: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Regeneron: Research Funding; Janssen: Research Funding; Cell Medica: Research Funding; Nanostring: Consultancy; FortySeven: Research Funding; Spectrum: Consultancy; Gilead: Consultancy; Bayer Healthcare Pharmaceuticals: Research Funding; Genentech: Research Funding; Agensys: Research Funding; Juno Therapeutics: Consultancy. Feldman: Kite Pharma: Speakers Bureau; Pharmacyclics: Speakers Bureau; Seattle Genetics: Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy; Janssen: Speakers Bureau; AbbVie: Speakers Bureau; Celgene: Speakers Bureau. Lansigan: Spectrum Pharmaceuticals: Consultancy, Research Funding; Seattle Genetics: Consultancy. Kahl: ADC Therapeutics: Research Funding; Celgene: Consultancy; Gilead: Consultancy; Seattle Genetics: Consultancy; Genentech: Consultancy. Casulo: Celgene: Research Funding; Gilead: Honoraria, Other: travel support; Infinity: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal